Revolutionary Nanomedicine
Nanomedicine operates at the forefront of modern healthcare, utilizing materials to encapsulate active drugs. This revolutionary approach to treatment have several advantages: 1) improved bioavailability; 2) targeted drug delivery; and 3) extended release and stability [1]. However, despite these promising features, the lack of precise, high-resolution analytical techniques capable of characterizing nanoformulations poses a challenge for their clinical approval due to their physicochemical complexity.
The Role of Fluorescence Lifetime in Nanomedicine
The 1st FDA-approved nanomedicine, Doxil®, encapsulates the active ingredient doxorubicin, a drug used for breast cancer treatment[2]. Notably, doxorubicin exhibits autofluorescence, making its nanoformulation to be analyzed through fluorescence lifetime analysis (FLA). A recent study revealed that encapsulated doxorubicin have three distinct physicochemical states [3]: 1) free-in-solution (left); 2) crystalline (middle), and 3) membrane-associated (right). Each state corresponds to a unique fluorescence lifetime , enabling precise control over drug quality through FLA.
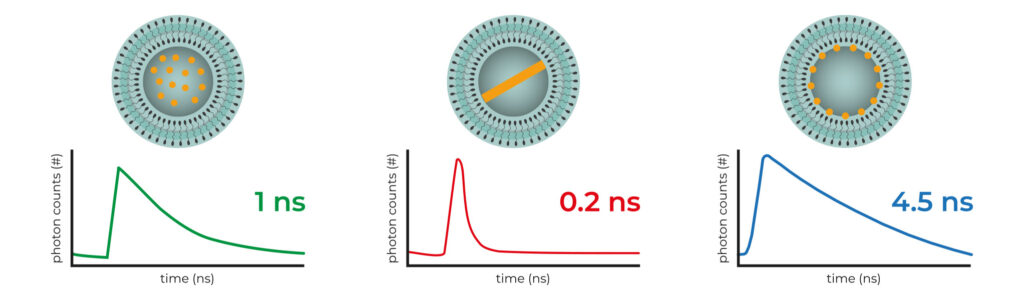
Three possible physicochemical states of encapsulated Doxorubicin and their associated fluorescence lifetime when excited at 488 nm
FLIM LABS’ Innovative Solution
At FLIM LABS, we have developed a compact, versatile, and portable FLA device for nanomedicine analysis.
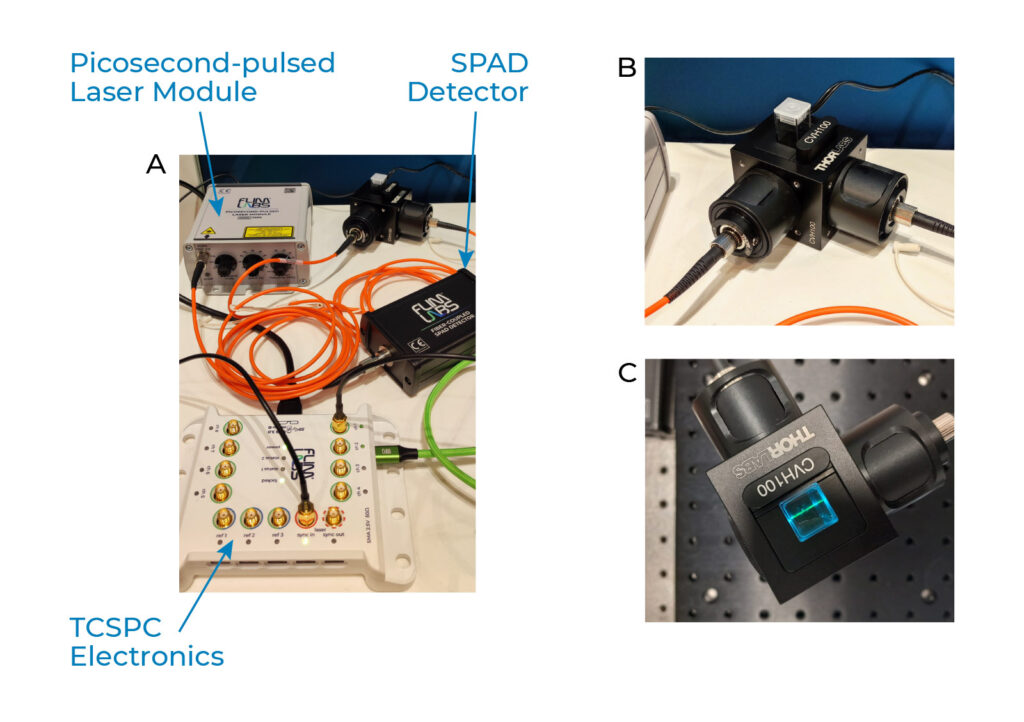
FLA device for drug quality control
It is based on the FLIM Starter Kit and involves a 488 nm picosecond-pulsed laser and a single-photon avalanche diode detector (SPAD) for precise fluorescence lifetime measurements. The temporal resolution is achieved through the FLIM Data Acquisition card, which reconstructs TCSPC histograms. Then we process the TCSPC data using the phasor plot approach, providing an intuitive means to extract valuable information. Importantly, the linear mathematical properties of the phasor plot also facilitate composition assessment.
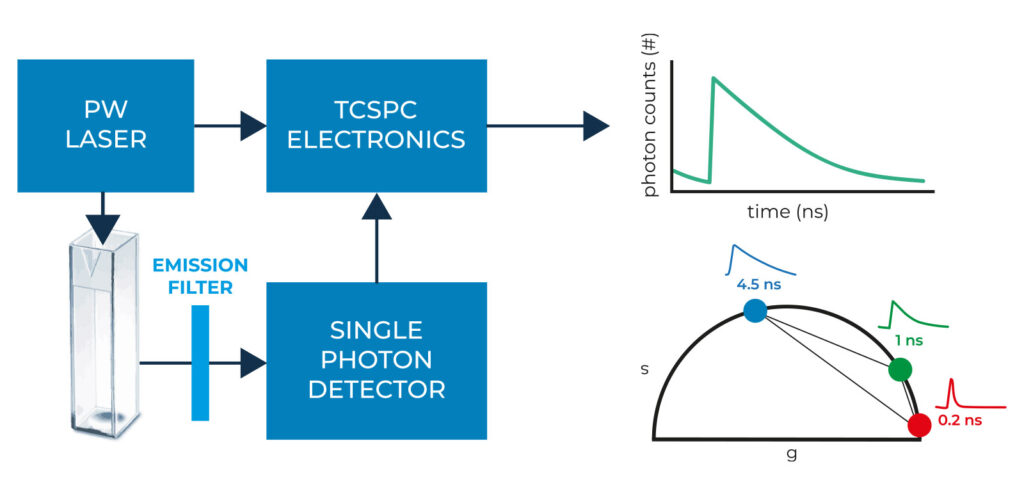
Working principle of the FLA system
Proven Performance
Our FLA System analyzed two batches of Doxil®, effectively assessing thier supramolecular organization within the nanoformulation. Our results suggested that the encapsulated doxorubicin exhibited a multi-exponential decay, attributed to the contribution of the three distinct states. Moreover, our results have confirmed the presence of variations among production batches, which poses a significant challenge in the clinical translation of nanomedicine [5].
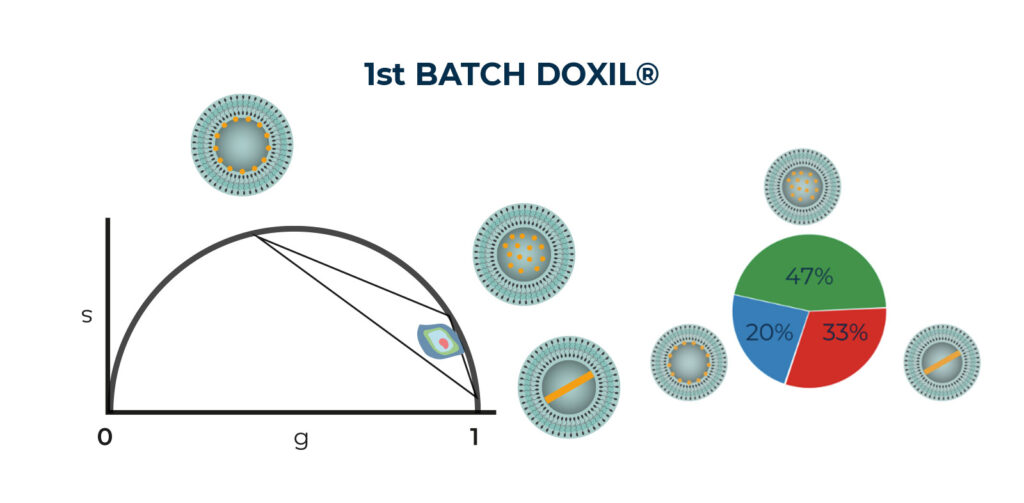
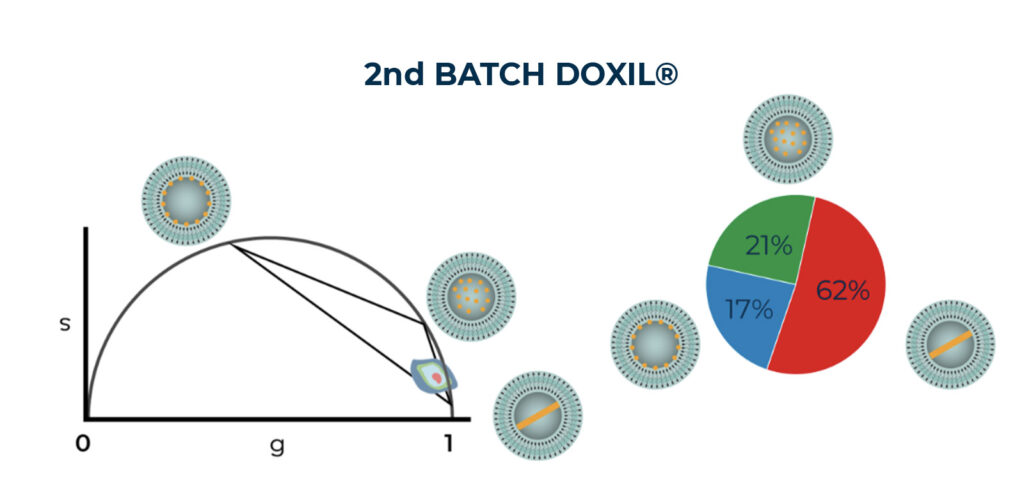
Phasor Analysis results of TCSPC data generated by FLA system and the proportionality of the three states based on phasor plot linear mathematical property
Our portable device not only meets the unique demands of nanomedicine analysis but also opens new avenues for quality testing along various production lines, including agrochemicals (e.g., controlled-release pesticides), industrial chemicals (e.g., paints, inks, cosmetics), and nutraceutical/dietary supplements.
Gratitude and Recognition
This work was supported by following funds: FLASH project for the valorization of patents through the Proof of Concept ”JUMP 2023” program (financed by MIMiT within the PNRR) from the European Union- Next Generation EU; project ECS00000017 ‘Ecosistema dell’Innovazione’ Tuscany Health Ecosystem (THE, PNRR, Spoke 4: Nanotechnologies for diagnosis and therapy) from the European Union-Next Generation EU
Bibliography
[1] Zhang, C., Yan, L., Wang, X., Zhu, S., Chen, C., Gu, Z., & Zhao, Y. (2020). Progress, challenges, and future of nanomedicine. Nano Today, 35, 101008.
[2] Fan, D., Cao, Y., Cao, M., Wang, Y., Cao, Y., & Gong, T. (2023). Nanomedicine in cancer therapy. Signal Transduction and Targeted Therapy, 8(1), 293.
[3] Tentori, P., Signore, G., Camposeo, A., Carretta, A., Ferri, G., Pingue, P., … & Cardarelli, F. (2022). Fluorescence lifetime microscopy unveils the supramolecular organization of liposomal Doxorubicin. Nanoscale, 14(25), 8901-8905.
[4] Rossetta, A., Bernardi, M., & Cardarelli, F. (2024, March). Fluorescence lifetime analysis (FLA) for the screening of healthcare nanoformulations: towards a compact and versatile device. In Reporters, Markers, Dyes, Nanoparticles, and Molecular Probes for Biomedical Applications XV (Vol. 12862, pp. 37-40). SPIE.
[5] Metselaar, J. M., & Lammers, T. (2020). Challenges in nanomedicine clinical translation. Drug delivery and translational research, 10, 721-725.











